Paradigm Shift in Technologies of Sample preparation for
Chemical Analysis
Electromembrane Extraction:Electric Membrane Extraction(EME)began with the idea of adding an electric field to hollow fiber liquid-phase microextraction and was standardized for research purposes by ETN in Norway, the first company in the world to do so.
EME Extraction Principle
Compounds that ionize in solution can in principle be extracted by EME.
1 Inject sample solution (donor solution) and acceptor solution into conductive vial
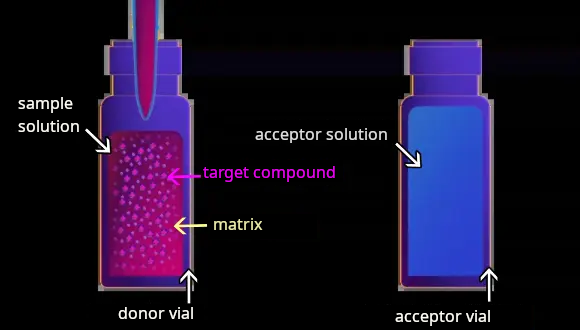
Sample solution (donor solution) and acceptor solution are injected into separate conductive vial such as a donor vial and an acceptor vial respectively. Since the target compound must be ionized in the solution, the type of target compound (basic, acidic, or zwitterionic) and its pKa value are important information for pH adjustment of the solution.
2 Constructing SLM
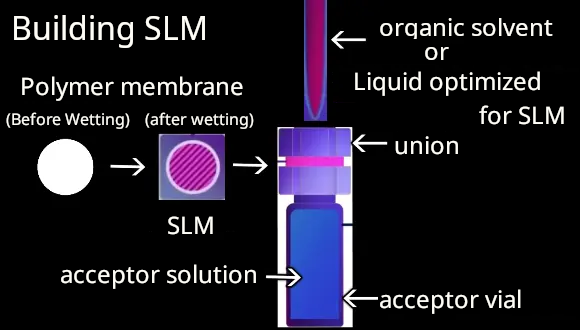
An union installed with a polymeric membrane is attached to an acceptor vial. Then, the SLM is constructed by dropping the small volume of appropriate organic solvent or optimized liquid membrane onto the polymer membrane for extracting the target compound.
*SLM stands for Supported Liquid Membrane. It is a composite membrane in which organic solvent infiltrates into the pores of the polymer membrane, supported and immobilized by the polymer fibers, and integrated into the membrane. Numerous reports have shown improved extraction efficiency when optimized according to the type of target substance (basic, acidic, or zwitterionic compounds) and chemical properties (structural formula, logP).
3 Connecting acceptor vial and donor vial
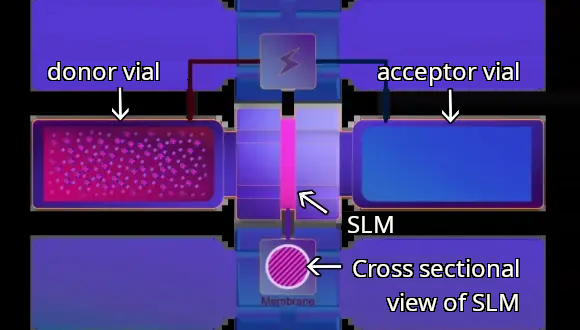
After the construction of SLM, the acceptor and donor vial are connected. The donor and acceptor solutions are separated by the SLM in the union. This twin vial unit is loaded into the ETN-12 EME system. 12 sets of twin vial units can be loaded into the device, allowing 12 samples to be extracted simultaneously.
4 Extraction with EME
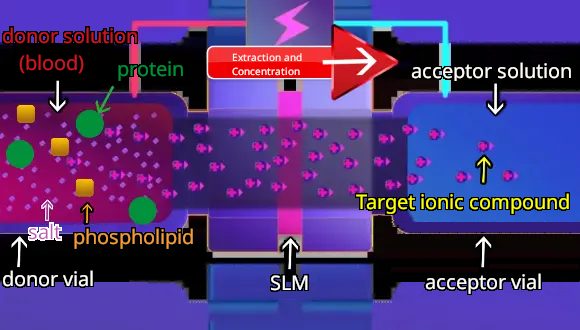
When voltage is applied, charged target ionic compounds are extracted from the donor solution to the acceptor solution through the SLM by electrical migration. Nonionic compounds and oppositely charged ionic compounds are retained in the donor solution. Electrolytes such as salts are also retained in the donor solution because the SLM is an organic solvent and hydrophobic. The hydrophobicity of SLM makes it difficult for hydrophilic salts to pass through the SLM. Thus, EME allows for highly selective extraction by optimizing the degree and direction of the voltage, pH of donor and acceptor solution, and composites of SLM.
5 Matrix-free purified extract
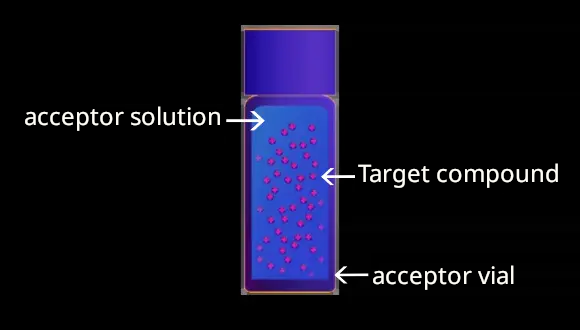
Extraction by EME yields pure sample extracts; EME provides very clean extracts under optimized conditions with reduced matrix effects, i.e., proteins and phospholipids are completely removed, reducing downtime of the MS instrument. Extracts can be injected directly into the LC-MS instrument using a very short LC method prior to MS. Of particular note is the ability of the ETN-12 EME vial system to complete the entire process from sample clean-up to solution preparation prior to analysis in a single step, without having to change vessels even once.
In fact, there have been numerous reports of compound extraction from blood samples using EME, and analysis by LC-MS has repeatedly demonstrated that extracts extracted with EME are matrix-free.
Short Movie of Compound Extraction with ETN-12 EME
Parameters of EME
The parameters of EME are shown below. Optimization of each parameter improves extraction efficiency.
Parameters to be considered and determined based on the information of the analyte prior to EME
- Criteria for determining if an analyte can be extracted with EME or not depends on whether the analyte ionizes.
- The direction of voltage application depends on the type of analyte such as basic compounds or acidic compounds or zwitterionic compounds.
- pH adjustment of donor and acceptor solutions depends on the type of compound above and the pKa value of analyte. It is considered a guideline to adjust the pH of the solution to 2 units above or below the pKa value of each compound. For example, if the target compound is a basic compound with a pKa value of 4, the buffer should be adjusted to a pH of 2, which is a strong acid. Conversely, if the target compound is an acidic compound with a pKa value of 6, the buffer is adjusted to a pH of 8, which is alkaline. In the case of zwitterionic compounds, past research reports have shown cases in which making the buffer acidic was effective. You can search for the first extraction conditions for your analyte at how-to-eme.com below.
- The optimization of SLM depends on the type of analyte (basic compounds, acidic compounds, zwitterionic compounds), structural formula of the analyte and the value of the pKa and logP.
The volume of organic solvent used to construct the SLM is 8 to 10 microliters. The membrane is approximately 10 mm wide and 0.1 to 0.3 mm thick. The organic solvent used to infiltrate the membrane depends on the extraction target. If the extraction target is a basic analyte, 2-nitrophenyl octyl ether (NPOE) is often used. On the other hand, if it is an acidic analyte, higher alcohols (hexanol, heptanol) are used. Ion carriers are sometimes added.
Parameters to be set by the device
- Voltage strength
- Direction of voltage application
- EME running time
- Stirring intensity setting During EME running, a boundary layer forms on the surface of the SLM membrane, which slows down the membrane transport of ions. To break it, the stirring intensity must be set.
A parameter to be monitored during EME running
- Electric current value during EME running In EME, the migration of ions generates a electric current. The current value is affected by the applied voltage, pH, addition of ion carriers, organic solvents for various SLM construction, and the amount of ions transferred from donor solution into acceptor solution. In EME extraction, the current value generated during running is a criterion to determine if EME has been successfully performed. Numerous past research reports and experimental data have proven that if the current value is less than 50 μA, no electrolysis or bubbles are generated in the system and EME is performed normally. The ETN-12 EME is equipped with a current value monitoring function that records the current value during EME running. If the current is too high, electrolysis of water, gas generation and pH change may occur sequentially. Therefore, the current must be maintained in the low microampere (μA) range for the successful extraction.
EME parameter setting
Please enter the name of compound in English in the search box on the following website to find out if the compound can be extracted with EME or not. If the compound can be extracted with EME extraction, recommended conditions for the first extraction of your analyte will be displayed. If the recovery rate is low, optimization will be necessary.

Advantages of EME
This innovative compound extraction technology, EME, has many advantages over existing pretreatment methods such as LLE (liquid-liquid extraction) and SPE (solid-phase extraction).
1 Rapid one-step completion from sample cleanup to sample preparation prior to analysis with analytical instruments
One-step solution to labor-intensive and costly pretreatment! Both proteins and phospholipids are completely removed simultaneously!
A target analyte can be extracted in a single step with EME from biological or environmental samples containing complex matrices. The process of protein precipitation and phospholipid removal using organic solvents are not necessary any more. Furthermore, our EME enables you to skip multiple steps such as concentration, evaporation to dryness and redissolution steps required for LLE and SPE. Thus, EME is a simple and effective technology for sample cleanup and preparation prior to the analysis with analytical instruments such as LC-MS and HPLC etc.
2 Matrix-free purified extracts are obtained
Easy, fast and matrix effect free!
As stated in the extraction principle of EME above, EME allows highly selective extraction by optimizing the extraction conditions. The removal of proteins, phospholipid, salts and opposite charged ions can result in a pure extract. Analytical data with MS have repeatedly proven highly effective to discriminate the matrix effect.
3 Role as a concentrator
Concentration is completed at the same time as extraction, allowing direct analysis!
By intentionally reducing the volume of the acceptor solution compared to the volume of the donor solution, the enrichment of target analyte can be achieved along with the sample clean-up and compound extraction. The solution can be analyzed directly with analytical instruments such as LC-MS without the reconstitution.
4 Promoting Greener Chemistry
Go for Greener Chemistry!
The volume of organic solvent used in EME is about 10 µl per sample needed for constructing SLM. This significantly reduces the amount of organic solvent used, which is harmful to user health and the environment, compared to existing LLE and SPE.
5 Non-destructive extraction
Capable of extracting compounds in a sample without destroying them!
The extraction principle of EME efficiently extracts ionized compounds in solution by electrochemical migration under normal temperature and pressure, and completes the concentration. Therefore, unlike existing pretreatment processes such as high-level homogenization and concentration at high temperature and pressure, EME can prepare samples for analysis under conditions that are mild for the compounds. Numerous reports have shown that EME has never been found to degrade or destroy compounds.
6 Zero risk of sample loss
Eliminate the risk of sample loss!
Unlike existing pretreatment methods, our product, ETN-12 EME, completes the entire pretreatment process in a single vessel, eliminating the risk of sample loss due to vessel replacement, evaporation, or adhesion to the vessel during concentration and evaporation to dryness. This advantage is especially maximized when extracting compounds from rare samples with small volumes.
7 Free from the stress of tedious and multiple steps of sample preparation
Ease sample preparation with a single step!
With EME, the pretreatment process usually requiring a great deal of time and effort can be completed in a single step. In addition, since a database is in place that allows anyone to easily search for information on the condition settings for each compound, there is no need to search the literature for EME condition settings. The combination of ETN-12 EME and our EME database standardizes whole process of sample preparation called as a craftsman’s job.
8 Agreement with Analytical Validation
Can be replaced with existing pretreatment methods!
The reproducibility of EME has proven to be high and complies with the level of analytical validation required by the FDA and EMA. Comparison of EME with existing routine methods shows that there is no inferiority to existing methods in terms of analytical data, and the advantages of EME are reconfirmed.
9 Does not contaminate columns or analytical instruments
Cut downtime and maintenance costs for analytical equipment!
Extracts obtained with ETN-12 EME do not contaminate columns or analytical instruments because matrices are completely removed. Frequent replacement of expensive columns, equipment maintenance costs, and downtime can be significantly reduced, contributing to more efficient analytical operations and research and development.
Research literature and chemical data showing the effectiveness of EMEs
- Recovery rate Actual data <In preparation>
- Simultaneous removal of protein and phospholipids <In preparation>
- Research report on Analytical Validation <In preparation>
Reasons to recommend EME
EME is ideal for extracting ionized compounds from biological and environmental samples such as blood and body fluids that contain multiple matrices.
- No need to change containers as is the case with LLE and SPE extractions
- Only ten microliters of organic solvents for extraction, contributing to greener chemistry
- Allowing for rapid extraction (3-15 minutes)
- Simultaneous removal of proteins and phospholipids for efficient sample cleanup
- Allowing for highly selective extraction
- Clean and pure extraction to eliminate or minimize the need for chromatographic separation
- Direct analysis of EME extracts with analytical instruments such as LC-MS due to automatic enrichment along with the extraction, requiring no further concentration and reconstitution
- Matrix effect free
- Performance in reproducible recovery rates and compliance with analytical validation
- Contamination free of columns or analytical instruments thanks to pure extract of EME
- Reducing the frequency of maintenance due to contamination, resulting in no downtime and extra costs
- Reducing stress of sample preparation on researchers
Why do researchers use EME?
This is a comment from a inventor of ETN-12 EME as a researcher.
Selectivity and Specificity
One of the key advantages of electromembrane extraction is its exceptional selectivity and specificity. Unlike solid-phase extraction (SPE) and liquid-liquid extraction (LLE), which rely on interactions between analytes and stationary phases or solvent phases, respectively, EME leverages both electromigration and membrane permeation mechanisms. This unique combination allows EME to selectively extract target analytes based on their physicochemical properties, minimizing interference from matrix components and enhancing analytical sensitivity.
Minimal Solvent Usage and Environmental Impact
EME offers a significant reduction in solvent consumption compared to traditional methods such as SPE and LLE. In SPE, large volumes of organic solvents are often required to elute analytes from the sorbent material, leading to considerable solvent waste and environmental impact. Similarly, LLE involves the use of two immiscible liquid phases, further exacerbating solvent usage. In contrast, EME operates with minimal solvent volumes, typically in the microliter range, thereby reducing solvent waste and aligning with principles of green analytical chemistry.
Rapid Extraction Kinetics
Time usage is of essence in analytical chemistry and EME excels in this regard with its rapid extraction kinetics. While SPE and LLE may require prolonged extraction times to achieve adequate analyte recovery, EME’s accelerated extraction process not only increases laboratory productivity but also minimizes the risk of analyte degradation, ensuring the integrity of analytical results.
Compatibility with Small Sample Volumes
EME offers compatibility with small sample volumes, making it ideal for applications where sample availability is limited. In contrast, traditional methods such as SPE and LLE may necessitate larger sample volumes to achieve sufficient analyte recovery. By requiring only a small volume of sample, EME reduces sample consumption and facilitates the analysis of precious or scarce samples, particularly in fields such as clinical diagnostics and forensic analysis.
Versatility and Adaptability
Another advantage of EME lies in its versatility and adaptability across diverse analytical applications. Whether analyzing pharmaceuticals, environmental samples, biological fluids, or complex food matrices, EME can be tailored to suit a wide range of sample types and analyte classes. Researchers have demonstrated the efficacy of EME in extracting various analytes, including drugs, metabolites, pesticides, and environmental contaminants, underscoring its versatility in analytical chemistry.
In conclusion, electromembrane extraction represents a paradigm shift in sample preparation, offering compelling advantages over traditional methods such as solid-phase extraction and liquid-liquid extraction. With its exceptional selectivity, minimal solvent usage, rapid extraction kinetics, compatibility with small sample volumes, and versatility, EME is poised to revolutionize analytical chemistry. As researchers continue to innovate and refine this technique, the adoption of EME is expected to accelerate, driving advancements in analytical science and enabling new discoveries across diverse fields.
EME has the potential to become responsible for the paradigm shift because the technology provides:
- Excellent reproducibility
- Pure extracts
- Green sample preparation method
Expectations for EME by the inventor of ETN-12 EME
Welcome to the future of sample preparation
One area where EMEs are expected to be implemented is in the clinical field.
In particular, it is considered ideal for clinical laboratory automation because of its simplicity of operation.
There is a huge range of sample preparation equipment and techniques in use in clinical laboratories today, and quite a few analytical chemists are eagerly awaiting to see what technology will be next.
They all wonder if they are using the right techniques or if they should be looking for better ways to do things.
Next, we have a choice. What should the budget be spent on?
- Should separation columns be used after pretreatment to reduce analytical instrument maintenance costs and avoid downtime?
- Do you want to do an appropriate and easy pre-treatment with no hassle?
Directly injecting a sample that has only been diluted or minimally pretreated directly into the column will significantly shorten the column lifetime and increase maintenance costs and downtime for the mass spectrometer.
Extracting 100% of the compounds of interest from biological samples containing the most difficult matrices (foreign components) is not an easy task.
Because two simple questions come to mind.
Did you really extract 100% of the analytes from this matrix?
If not, can the results of the analysis be trusted? Can the results of this analysis be used to determine whether someone lives or dies?
A significant number of analytical chemists agree that there is a need for a paradigm shift in pretreatment technology. And the biggest players in the industry have understood the message and have decided to invest heavily in the future to be responsible for this projected paradigm shift.
EMEs have the potential to shoulder the responsibility for this paradigm shift.
Because, unlike existing cumbersome pretreatment methods
- This is because it enables rapid, 100% clean extraction from any biological sample in a single step.
- Valuable samples are not destroyed and can be extracted again and again.
- Extracts can be injected directly into a high-resolution mass spectrometer.
- No additional chromatographic separation is required unless there is further benefit after EME extraction.